Company Profile
Kossel Medtech (Suzhou) Co., Ltd. with the headquarter in Suzhou China, was established by experts in the US and domestic R&D team in 2013. Kossel Medtech focuses on R&D, manufacturing, and sales of Class 3 interventional cardiovascular medical devices, including products for peripheral vess, electrophysiology, coronary and technical service. Kossel Medtech's main clients are overseas and domestic high-end medical devices manufacturers, distributors, and hospitals.
Kossel Medtech (Suzhou) Co., Ltd. with the headquarter in Suzhou China, was established by experts in the US and domestic R&D team in 2013. Kossel Medtech focuses on R&D, manufacturing, and sales of Class 3 interventional cardiovascular medical devices, including products for peripheral vess, electrophysiology, coronary and technical service. Kossel Medtech's main clients are overseas and domestic high-end medical devices manufacturers, distributors, and hospitals.
OEM/ODM services:
1. Laser cutting (316L/ L605/304T/ Nitinol/MP35N etc.)
2. Heat treatment
3. Electrochemical polishing
4. Stents griping
Stent Size Matrix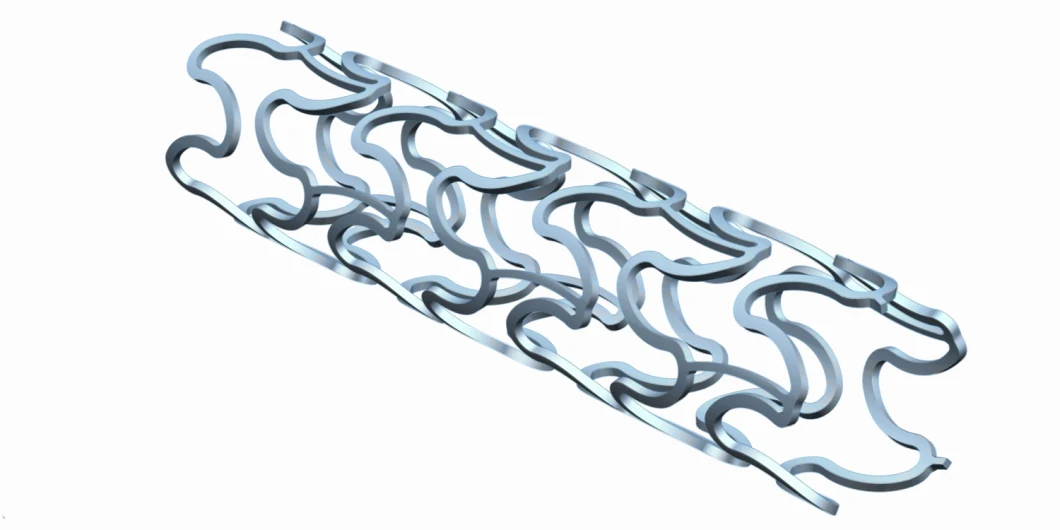
| L/mm D/mm | 8 | 12 | 13 | 15 | 16 | 18 | 20 | 21 | 23 | 24 | 28 | 29 | 33 | 38 | 40 |
| 2.0 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | - | - | - | - | - |
| 2.25 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | - | - | - | - | - |
| 2.5 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2.75 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 3.0 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 3.5 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 4.0 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 4.5 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 5.0 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
ISO 9001 13485 Certificate
Production Line
Contact Information